 Share on Facebook
Share on Facebook
 Sanofi will acquire Synthorx Inc to expand its immuno-oncology pipeline with THOR-707, an optimised IL-2 candidate.
Sanofi will acquire Synthorx Inc to expand its immuno-oncology pipeline with THOR-707, an optimised IL-2 candidate.
Sanofi SA‘s and Synthorx Inc's Boards approved the deal which forsees that Sanofi will acquire all of the outstanding shares of Synthorx for $68 per share in cash. The deals represents a 172% premium to Synthorx’s closing price on December 6, 2019, and represents a company value of roughly US$2.35bn (€2.6 bn). Sanofi expects to complete the acquisition in the first quarter of 2020.
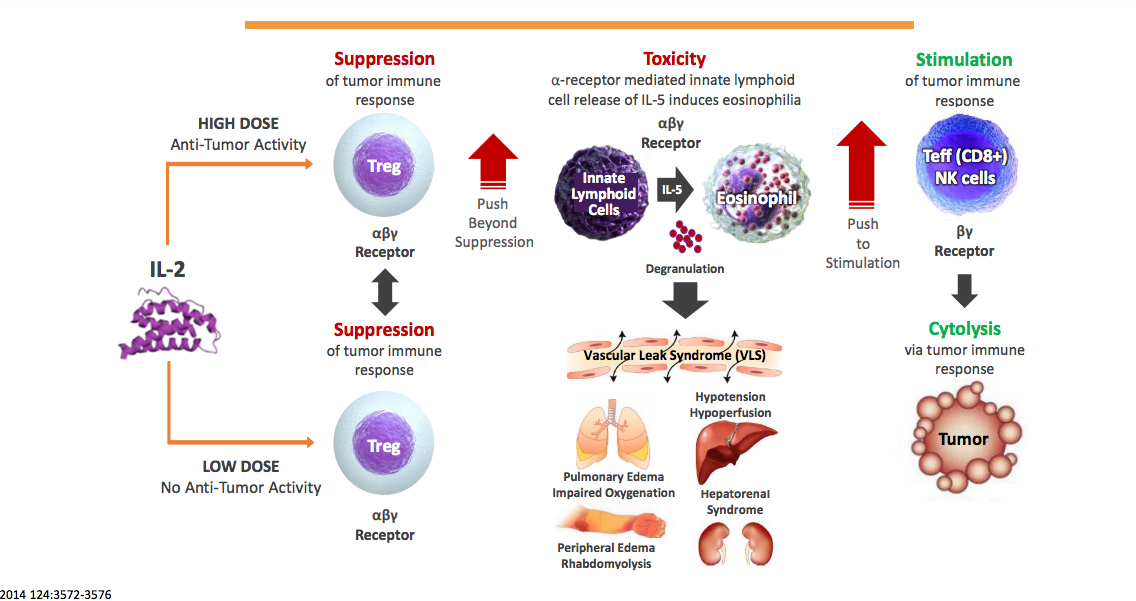
Synthorx Inc. is focussed on the development of drug for cancer and autoimmune disorders. Synthorx’s Expanded Genetic Alphabet platform technology develops so-called synthorins, proteins optimized through incorporation of novel amino acids encoded by an additional synthetic DNA base pair that enables site-specific modification, which improve the proteins druggability. The company’s lead immuno-oncology product candidate, THOR-707, is a pegylated IL-2 that prevents vascular leakage syndrome through blocking of IL-2 at its alpha-receptor (no alpha IL2), even when applied in high dose, and that has and extended half-life. The modified IL-2 boosts CD8 killer cell numbers in hematological and solide cancers and is developed as single agent and in combination with an immune checkpoint inhibitor. Synthorins were initially developed by by Dr. Floyd Romesberg and The Scripps Research Institute.
“This acquisition fits perfectly with our strategy to build a portfolio of high-quality assets and to lead with innovation," commented Paul Hudson, Chief Executive Officer at Sanofi. “By selectively expanding the numbers of effector T-cells and natural killer cells in the body, THOR-707 can be combined with our current oncology medicines and our emerging pipeline of immuno-modulatory agents for treating cancer. Moreover, Synthorx’s pipeline of engineered lymphokines has great promise not only for oncology but also for addressing many autoimmune and inflammatory diseases. ” added John Reed, Global Head of R&D at Sanofi.
THOR-707 has just entered Phase I safety testing in solid tumours and is in preparation for IND in combination with checkpoint inhibitors. The company is in lead optimisation of IL-10 and IL-15 synthorins. Sanofi announced it will combine THOR-707 with its drugs targeting PD-1, CD-38, and molecules that modulate effector T-cells and natural killer cells.



